GLOW conducts NLPHL research aimed at optimizing the diagnosis, care, and outcomes for patients of all ages diagnosed with NLPHL worldwide.
Please review the GLOW publication policy before completing a GLOW project application form. If you are not currently a GLOW researcher and would like to do research with GLOW, please email GLOW@urmc.rochester.edu to discuss our research processes.
GLOW maintains the world’s largest database of clinical data from over 2,000 patients of all ages and stages who have been diagnosed with NLPHL. As of 2022, GLOW is partnering with the NODAL and the Pediatric Cancer Data Commons to harmonize and house incoming retrospective clinical trials data. To learn more about how your site can contribute NLPHL case data to the GLOW retrospective database via NODAL, please contact Suzi Birz (sbirz@bsd.uchicago.edu) or email GLOW@urmc.rochester.edu.
The GLOW NLPHL biobank resides at Stanford University. Researchers who are interested in sharing or utilizing samples for collaborative research with GLOW may contact GLOW@urmc.rochester.edu to discuss.
NLPHLPRO is the first study of patient-reported outcomes in patients diagnosed with NLPHL. This multi-site GLOW study was designed by the GLOW Patient-Reported Outcomes Committee under the leadership of Dr. Ajay Major.
Valerie Crabtree, PhD | St. Jude Children’s Research Hospital
Anna Jones, PhD | St. Jude Children’s Research Hospital
Ajay Major, MD, MBA | University of Colorado School of Medicine
Jamie Flerlage, MD, MS | University of Rochester
Matthew Rees, MD | St. Jude Children’s Research Hospital
Dana-Farber Cancer Institute & Harvard University
Emory University
Hackensack University Medical Center
Memorial Sloan Kettering Cancer Center
Stanford University
St. Jude Children’s Research Hospital
University of Colorado
University of Rochester Medical Center
Additional non-GLOW NLPHL studies may be available. Below are organizations that maintain databases of active and historical clinical research.
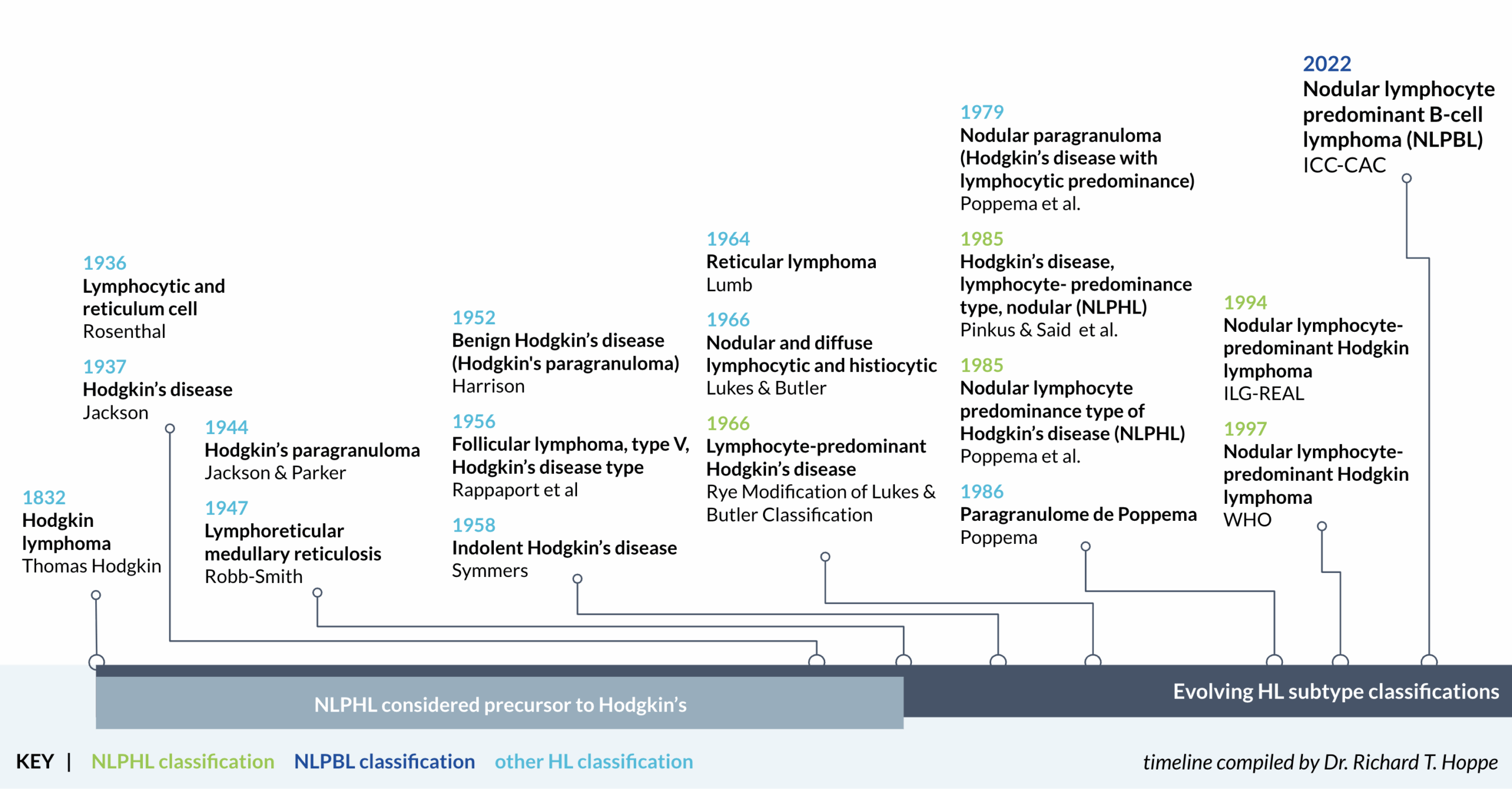
Thomas Hodgkin identifies Hodgkin lymphoma.
Jackson & Parker describe Hodgkin’s paragranuloma (NLPHL) as a distinct clinical entity. NLPHL is no longer considered “early Hodgkin’s.”
Lukes & Butler establish basis for modern classification of HL.
Regula et. al conclude NLPHL may have significant clinical and immunophenotypic differences from other Hodgkin lymphomas. Independent research groups conduct pilot studies of de-intensified treatment for NLPHL.
The World Health Organization recognizes NLPHL as a distinct subtype of Hodgkin lymphoma.
Fan et. al identify 6 histopathologic NLPHL variants.
Increasing understanding of differences in clinical outcomes between (rare) NLPHL and (more common)
cHL as well as a difference in expression of cell surface antigens leads to the exclusion of patients with NLPHL
from frontline HL clinical trials. Researchers worldwide are unable to secure funding for frontline NLPHL clinical trials.
The Global nLPHL One Working Group (GLOW) is established.
The International Consensus Classification (ICC) Clinical Advisory Committee (CAC) reclassifies NLPHL to nodular lymphocyte predominant B-cell lymphoma (NLPBL). The World Health Organization continues to use the term NLPHL.
Binkley and Flerlage et al. publish the largest retrospective analysis of NLPHL to date and create the lymphocyte-predominant international prognostic score (LP-IPS) [GLOW publication].
Major et al. publish the first study of patient and care partner perspectives on treatment decision-making in NLPHL [GLOW publication].
Palese et al. publish the GLOW Research Roadmap, a strategic action plan to overcome historical research challenges, establish a research pipeline to inform a global standard of care, and disseminate findings [GLOW publication].
The Global nLPHL One Working Group is committed to improving outcomes for all patients diagnosed with nodular lymphocyte-predominant Hodgkin lymphoma.
Interested in joining GLOW? Complete our Tell Us About You form!